Background
Myasthenia gravis is a rare, neuromuscular (disease of the muscle and nerves) autoimmune disease characterized by weakness of the muscles. MG has a prevalence of approximately 14–40 per 100,000 people in the United States. There is no cure for MG, however understanding the disease is crucial to pave the way for development of new therapies. Important to note, the most common subtype of MG associated with Acetylcholine receptor antibodies (AChR) are found in more than 80% of patients with generalized myasthenia gravis, whereas muscle-specific kinase (MuSK) antibodies are found in only 8% of MG patients. These are found in patients with the AChR antibodies.
Since the discovery of MuSK antibodies in patients, much has been learned. It has become clear that MuSK antibody MG differs in many ways from AChR antibody MG. There is some information that specific genetic factors play a role in development of MuSK MG.
About this Study
Our objective is to collect saliva samples from 1000 subjects with laboratory confirmed diagnosis of MuSK myasthenia. These saliva samples will be then sent to the laboratory of Bryan Traynor who directs the Neurogenetics Laboratory at NIH. Dr. Traynor will conduct a genome-wide association study (GWAS). This study will provide important information of genetic factors leading to MuSK MG.
What is Genome-Wide Association Study (GWAS)?
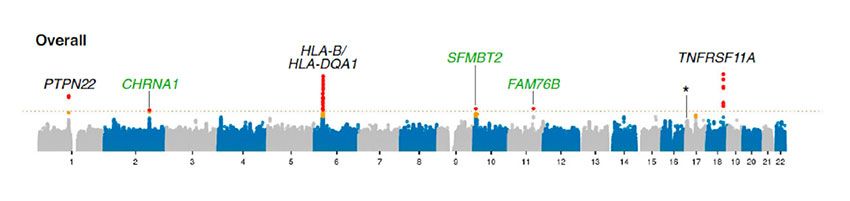
doi: 10.1073/pnas.2108672119)
GWAS, also known as genome-wide association study, is a study performed to identify genetic characteristics associated with a particular disease. GWAS is currently used across various disciplines in medicine to better understand complex conditions where genetic risk factors contribute to the development of diseases.
The image above shows the results of a GWAS study of 1,873 patients with subtype of MG known as acetylcholine receptor antibody-positive MG (AChR MG) and 36,730 controls (patients without MG). The genes shown are related to immune function and the primary antibody target of acetylcholine receptor antibody positive MG.
Presently, this study has not been done in patients with MuSK MG. We can use information from this study to develop quality treatment and ultimately, move toward targeted therapy approach for patients. We hope to find unique results and further enhance myasthenia research.
Eligibility
- Lab test confirming diagnosis of MuSK myasthenia gravis
- Willingness to provide saliva sample via mail or in person
Participation
In order to participate, please contact the study team by phone or by email. You can reach us at musk1000 [at] mfa [dot] gwu [dot] edu (musk1000[at]mfa[dot]gwu[dot]edu) OR 202-677-6109.
MuSK Myasthenia 1000
Department of Neurology & Rehabilitation Medicine
George Washington University
2150 Pennsylvania Avenue, NW
Washington DC 20037